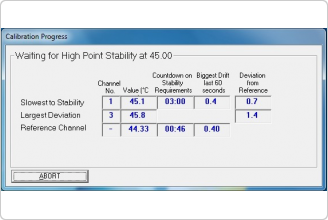
Description
TQSoft
• Compliance with United States Food and Drug Administration (FDA) Title 21 CFR Part 11 regulations on electronic records and signatures for incubation, sterilization, freezing, drying and temperature mapping validation applications in pharmaceutical and biomedical industries.
• Developed in accordance with Good Automated Manufacturing Practice (GAMP) from the International Society for Pharmaceutical Engineering (ISPE).
• Compliance with European standards for sterilization, decontamination, and disinfecting (EN554, EN285, EN15883, HTM2010, HTM2030), ISO 15833 requirements for washer-disinfectors, and ISO 17025 competence requirements for testing and calibration laboratories.
• Has been audited by major pharmaceutical companies and its quality documentation has passed FDA audits.
TQAero
• Compliance with National Aerospace and Defense Contractors Accreditation Program (NADCAP) and SAE International AMS 2750 guidelines covering industrial heat treating applications in aerospace and transportation industries.
• Supports heat treatment processes validation by Temperature Uniformity Survey (TUS) and System Accuracy Test (SAT) procedures required by AMS 2750.